FAQ's
FAQs: HUMANOID, Organoid Models, and Access to Human Tissues
1. What is HUMANOID?
HUMANOID (Human Multi-Omic Atlas Network for Organoid and Integrated Discovery) is a cutting-edge Center dedicated to advancing the fidelity and impact of Human Models of Disease. Housed within the UC San Diego Institute for Network Medicine—and supported by three transdisciplinary Centers—HUMANOID pioneers the creation of trademarked HUMANOID™ systems: miniature, biologically faithful models built by humans, from humans, for humans. Visit “Research” page to check out our disease models and FAQ #3 for how HUMANOID serves as a ‘living biobank’.
At its core, HUMANOID is driven by a bold mission—to harness the power of patient-derived organoids, often co-cultured with primary cells, and translate them into transformative breakthroughs in diagnostics, therapeutics, and precision medicine.
With deep expertise spanning organoid biology, primary cell systems, gene editing, high-resolution imaging, multi-omics, and machine learning, HUMANOID exists to decode disease mechanisms—and, ultimately, to help heal.
Founded in 2019, HUMANOID is located in the George Palade Laboratories, at the heart of UC San Diego’s main campus in La Jolla. The Center occupies approximately 2,000 square feet on the second floor and includes dedicated tissue culture facilities designed for both "clean" (sterile) cultures and "dirty" models that require co-culture with microbes—enabling a wide range of physiologically relevant disease modeling.
2. What are Organoids and Primary Cells?
- Organoids are 3D miniature tissue models grown from stem cells that mimic key structural and functional features of human organs. They offer unprecedented insight into development, disease, and therapeutic response—bridging the gap between cell lines and patients. Organoids can be derived from two sources: (i) induced pluripotent stem (iPS) cells vs. (ii) adult stem cells. There are pros and cons for each. See FAQ #7 for a breakdown that reflects current understanding in translational biology, disease modeling, and regenerative medicine. See FAQ #3 for how HUMANOID’s organoids extend as a ‘living biobank’.
- Primary cells are directly isolated from human or animal tissues and retain the physiological characteristics of the donor. They are essential tools for studying patient-specific biology in vitro, especially when maintained in optimized, biologically relevant conditions.
Together, organoids and primary cells allow us to model complex biology in a dish—with relevance to real-world human health. See answer to FAQ #5 on how we approach disease modeling with interdisciplinary agility to ensure model fidelity.
3. Are there Health Science or University-level efforts to support integration of organoid models into research?
UC San Diego is a leading hub for innovation in organoid technology [link], with faculty across nearly every medical subspecialty leveraging these models to discover new cures, biomarkers, and mechanistic insights into human disease.
Many campus investigators specialize in organoids derived from induced pluripotent stem (iPS) cells—such as brain (Muotri, Yeo), heart (Chi, Sheikh), lung (Liebel), and liver (Kisseleva). Others, including the HUMANOID™ Center, focus on organoids derived from adult stem cells. (See FAQ #7 to explore which organoid model best suits your research.)
Researchers like Fraley, Friend, and Schöneberg are advancing the field through technological innovations in organoid culture, propagation, and enhancement—including the integration of microfluidics and other next-generation tools.
This list is neither comprehensive nor exhaustive, but it highlights the breadth and depth of organoid-related expertise on campus.
At present, HUMANOID is the only formal organoid-focused center at UC San Diego. Though housed within the Department of Cellular and Molecular Medicine, HUMANOID functions as a cross-campus hub—supporting research across disciplines, divisions, and even institutions, both nationally and internationally.
4. What kind of support does HUMANOID offer?
- Infrastructure:
Access to high-end equipment, a patient-derived biobank, Metadata repositories, IRB protocols, and 2,500 sq. ft. of lab space in the George Palade Laboratories. Dedicated tissue culture rooms and appropriate biosafety levels (BSLs) support both sterile and pathogen-inclusive (microbe/pathogen) models.
- Funding:
While launched on limited resources, HUMANOID now operates sustainably. It offers a Pay-It-Forward voucher program to help faculty generate preliminary data for grant applications. This program is limited and discretionary, aligned with HUMANOID’s internal priorities and available funding.
- Staffing:
Operates with 8 FTEs, including a Director who can serve as co-investigator on grants and manage regulatory documentation, MTAs, CDAs/NDAs, and other compliance-related activities.
- Training:
Offers hands-on organoid training modules and can serve as a host site for practical instruction in degree-granting programs (undergraduate and graduate) in Stem Cell Biology.
- Grant Submission:
The Director is enabled to join grants and carry budgets through CMM. HUMANOID can provide all necessary documentation: biosketches, other support, facilities and resources, budget/justification, equipment descriptions, human subjects protection plans, and letters of support.
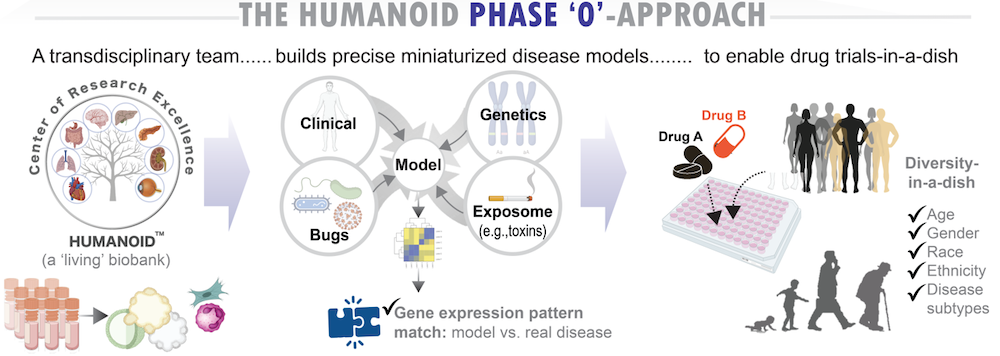
5. What is a ‘living biobank’?
HUMANOID strives to maintain organoids as living extensions of the patients from whom they are derived—creating a dynamic, ‘living biobank.’ This allows us to not only model disease features with fidelity but also to ask whether organoid phenotypes predict real-world patient outcomes, and whether drugs that show efficacy in organoids may one day benefit the individuals they represent.
6. How does HUMANOID approach disease modeling?
HUMANOID operates by a clearly defined ethos rooted in the principles of disruption through innovation, open science and collective progress. We don’t play by the rules; we make them. Our values include:
- Prioritize Impact Over Redundancy When Biobanking Organoids:
We avoid duplicating standard models and instead focus on high-impact, underexplored therapeutic areas. Our biobank includes leading-edge models in inflammatory bowel disease (IBD), idiopathic pulmonary fibrosis (IPF), hereditary cancer syndromes, atherosclerosis, and immunotherapy response.
- Collaborate, Don’t Compete:
We honor existing expertise across campus and work to complement rather than replicate. When gaps arise, we co-develop models that fill those needs—like complex cardiac organoids or fibrosis models.
- Embrace Interdisciplinary Integration:
At the heart of our modeling philosophy is a bidirectional process: we simplify to understand and then rebuild complexity to predict and test. Our models are tightly integrated with tools from engineering, computational science, and systems biology—not to mimic biology superficially, but to distill disease to its molecular essence. By stripping away the superfluous, we create streamlined, multi-cellular 3D models that capture the most outcome-determinative features of disease—genes, proteins, metabolites—that correlate with real-world clinical endpoints. From these simplified yet rigorous constructs, we then reintroduce complexity digitally. Through mathematical modeling and in silico simulations, we explore emergent behaviors and test therapeutic perturbations virtually—reconstructing the disease system to ask new questions. Wherever possible, these simulations are anchored to ground truth: patient outcomes, radiographic patterns, epidemiologic metrics. This loop—of abstraction and reconstruction—is central to our ethos and is embraced in our signature program, BIODESIGN. Inspired by the engineering precision of biological systems, we believe that to build is to understand, and to understand is to build. Through this wet-lab–digital synergy, we accelerate discovery, translation, and impact. To learn more about BIODESIGN, click here.
- Model with Rigor and Yet, aim for Simplicity:
Our BIODESIGN program is guided by a principle echoed in Pablo Picasso’s approach to abstraction: the stripping away of the superfluous to arrive at the essential. Just as Picasso distilled form to capture deeper truths in art, we apply computational abstraction and rigorous benchmarking to ensure our models faithfully recapitulate the core biology of the diseases they aim to mimic. By focusing on what truly matters—and eliminating noise—we build models that are not only scientifically sound but also functionally relevant. To learn more about BIODESIGN, click here.
- Tie Models to Patient Outcomes:
Many of our models are derived from prospectively followed clinical samples, allowing validation of therapeutic effects against actual patient trajectories. Some efforts support “Phase 0” organoid trials as precursors to Phase 2a/b clinical trials.
- Reimagine Possibility:
Modeling disease without a clear understanding of the key drivers linked to patient outcomes—such as progression risk, therapeutic failure, or adverse events—can feel like shooting in the dark. At HUMANOID, we see this uncertainty not as a limitation, but as an invitation to innovate. It demands an approach grounded in humility, anchored to clinical reality, and guided by both precision and imagination. By challenging assumptions and embracing unconventional ideas, we uncover solutions others may overlook. To learn more about our signature BIODESIGN approach, click here.
- Share Freely and Transparently:
Our protocols, media and reagents, and methodologies are open-access. Our media is already shipped world-wide and can be purchased directly from the Center. We prioritize reproducibility and global dissemination. HUMANOID’s goal is not to own the standard—it is to be the standard by enabling others. Organoids, however, may not be shared unless originating IRB (patient consent) and the contributing clinician(s) explicitly permit such sharing, and regulatory approvals (e.g., MTA’s for inter-institution sharing or licensing for sharing with for-profit entities) are in place.
- Accelerating Discovery with Precision, Integrity, and Respect for Humanity:
At HUMANOID, we unite speed and scientific precision with an unwavering commitment to the highest ethical standards in human subjects research. Guided by humility and respect for the individuals behind every sample, our expansive IRB framework enables broad access to tissues—from healthy donors to those with chronic or infectious diseases. By serving as an "umbrella" protocol, our IRB connects multiple clinics, investigators, and patient populations, streamlining access to fresh or viably frozen tissues for organoid generation. Through seamless reciprocity between IRBs—with consent that explicitly enables the creation of expandable human models—we make it possible to launch new organoids without delay, accelerating innovation while honoring patient trust.
7. Can HUMANOID help isolate and culture mouse organoids?
While HUMANOID’s primary focus is on human-derived models, our team has deep expertise in isolating and culturing murine (mouse) organoids as well. We can assist with protocol development, training, and pilot studies involving mouse tissue, particularly if the work contributes to translational research goals or supports human-model system comparisons.
Please reach out to discuss feasibility and scope—we are happy to explore how we can support your project.
8. How do I know which type of organoid is best for my research program?
Organoids can be derived from Induced pluripotent stem cells (iPSC) adult stem cells (AdSC). Each model type has unique strengths and limitations, and the choice depends on the research question at hand.
iPSC-derived organoid models offer the advantage of limitless expansion and the potential to differentiate into nearly any cell type, making them ideal for organ modeling, studying tissue development and or rare genetic diseases, genetic manipulation, and high-throughput drug screening. However, they lack epigenetic memory of the diseased tissue or its environment, require complex differentiation protocols (which can negatively impact reproducibility), and may not fully replicate mature adult tissue behavior.
In contrast, AdSC-derived models are typically more stable and closely reflect tissue-specific functions, architecture, and disease-driving environmental factors, making them valuable for translational research and personalized medicine. Yet, they are limited by greater challenges in genetic modification.
AdSC-derived models exhibit inter-individual variability, with cell states influenced by factors such as age, health, and environment. However, this is not a limitation when supported by a sufficiently large and diverse biobank—an asset that reflects real-world human heterogeneity far better than inbred, isogenic mouse models. In fact, many drug discovery programs fail due to lack of efficacy in diverse patient populations in Phase 3 clinical trials, underscoring the need for models that capture this variability. HUMANOID’s extensive and diverse biobank is purposefully designed to overcome this gap and accelerate the discovery of truly effective therapeutics.
9. What are the recent developments (guidelines, regulatory approvals) in organoid research that I should be aware of?
Merck KGaA’s recent acquisition of HUB Organoids—a spin-off from the Hans Clevers lab at the Royal Netherlands Academy of Arts and Sciences, where foundational intellectual property for creating patient-derived organoids (PDOs) was developed—strategically positions the company to accelerate the development of PDO technology, a critical enabler for drug discovery. This move aligns with significant regulatory shifts in the United States aimed at modernizing drug development processes.
In September 2022, the U.S. Congress passed the FDA Modernization Act 2.0 (S. 5002), which permits the use of non-animal testing methods, such as organoids and computer modeling, to establish drug safety and efficacy. This legislation marks a pivotal shift towards more human-relevant testing methods in the pharmaceutical industry.
More recently, in April 2025, the U.S. Food and Drug Administration (FDA) announced a strategic roadmap to phase out animal testing requirements for monoclonal antibodies and other therapies. The FDA plans to promote the use of lab-grown human organoids and organ-on-a-chip systems that mimic human organs, aiming to provide more accurate predictions of human responses and reduce reliance on animal models.
Concurrently, the National Institutes of Health (NIH) has launched an initiative to prioritize human-based research technologies. Under the leadership of NIH Director Dr. Jay Bhattacharya, the agency is integrating advances in data science and technology with a growing understanding of human biology to reimagine the way research is conducted, moving away from traditional animal models.
These developments underscore a significant shift in biomedical research and drug development, emphasizing the importance of human-relevant models like PDOs. HUMANOID’s extensive and diverse biobank is purposefully designed to support this transition, enabling more accurate and efficient discovery of therapeutics that are effective across diverse patient populations.
10. What is HUMANOID doing to make organoid research accessible and affordable?
Organoid research is a powerful but resource-intensive endeavor. The high cost stems from a combination of factors, including:
- Specialized skills and training required to grow and maintain organoids
- Expensive reagents such as defined media, Matrigel, growth additives, and specialty cultureware
- Lengthy quality control processes to ensure organoid fidelity and reproducibility
- High failure rates without optimized protocols and prior experience
- Limited access to diverse, well-characterized biospecimens
HUMANOID is tackling these affordability and accessibility barriers through a multipronged approach:
- A Robust Biobank of Living Organoids: HUMANOID houses a growing, diverse biobank of patient-derived organoids from various tissues, disease states, and demographics. By providing ready-to-use, validated models, we eliminate the time and cost of in-house derivation.
- Low-cost Media Solutions: HUMANOID leverages standardized SOPs that primarily use L-WRN conditioned media—a cost-effective and widely validated alternative to expensive commercial defined media. This approach enhances reproducibility across laboratories while significantly reducing costs. By minimizing the use of exogenous additives, HUMANOID not only cuts expenses but also seeks to preserve the cell-autonomous properties essential for establishing native growth factor gradients and tissue-specific niches. We support media production and distribution both nationally and globally to broaden access.
- Training and Education Hub: HUMANOID provides both hands-on wet lab training and theoretical workshops for researchers at all levels. Our immersive education model demystifies organoid technology and democratizes its use across disciplines.
- Voucher Program – Pay it Forward: Through a “Pay It Forward” voucher program, supported by philanthropic and institutional partnerships, we help offset research costs for junior investigators, trainees, and resource-limited labs. Vouchers can be used for services, reagents, or training, and eligibility for it is decided on a case-by-case basis.
- Collaborative Research Model: By integrating across multiple Centers within the UC San Diego Institute for Network Medicine, HUMANOID fosters team science that reduces duplication of effort and shares discoveries across labs and disease areas.
HUMANOID is committed to making next-generation human models of disease available to a broader scientific community, accelerating innovation while lowering barriers to entry.
11. Can we get a vial of organoids? *or* How do I get organoids incorporated into my research if my lab lacks expertise in organoid handling?
Because the foundational conceptual and technological intellectual property (IP) that goes into the derivation of PDOs is not owned by us (see FAQ #9, above), we are not permitted to hand over propagatable vials of cells as a “sale,” with one key exception: when the contributing physician or surgeon is directly involved in organoid research within their own group. This exception is possible because the costs on both sides are defrayed within a collaborative framework, grounded in an honor system. More broadly, we do not have a license to sell organoids, which limits our ability to distribute them in this way.
That said, we routinely share endpoint materials such as DNA, RNA, cell lysates, fixed cells, FFPE blocks, or cells ready for fixation, and terminally differentiated monolayers—for instance, when there’s a need to study the gut barrier. While we are fully equipped to run these assays in-house, we encourage PIs to perform these endpoint studies in their own labs whenever possible, especially if they have the personnel and expertise. This helps reduce the need for recharge and supports cost-effective collaboration. We also routinely send organoid end point assays to various on-campus cores (e.g., the IGM core for RNA Seq, LJI core for histopathology of embedded PDOs in histogel, made into FFPE blocks for subsequent IHC/IF; or Proteomics core for total and phosphoproteomics, etc). We have established partnerships and streamlined workflows with nearly every major core on campus.
Finally, in broader collaborations—where we are engaged as a partner or listed as key personnel—we routinely engage our computational team to analyze large scale datasets (e.g., RNA Seq, proteomics, scRNA Seq, etc) to extract insights from information using the unique off-the-beaten-path capabilities within the Center for PreCSN.
This integrated approach allows investigators to leverage HUMANOID as a one-stop resource for organoid-related studies, from biological materials to data interpretation.
12. Does HUMANOID provide training, or share standard or organoid type-specific set of protocols for organoid research?
HUMANOID offers comprehensive training modules covering all aspects of organoid work.
For labs interested in building in-house expertise in organoid derivation and culture, we provide immersive, hands-on training modules—typically over a two-week period. These sessions are designed to support PIs, fellows, and students in learning how to establish, expand, maintain, passage, freeze, and otherwise manipulate mouse or human patient-derived organoids (PDOs).
For labs capable of performing endpoint assays (such as those listed in FAQ #11) but lacks experience with organoid handling techniques—such as imaging, staining, fixing, or extracting from Matrigel—we are happy to offer targeted training and/or share validated protocols to support those needs.
For IP that is generated within HUMANOID (i.e., modeling of diverse diseases using PDOs), we publish detailed protocols to ensure that ‘open access’ is prioritized.
13. How can I access HUMANOID’s services or schedule a consultation?
Accessing HUMANOID’s capabilities is easy. We offer:
- Consultations for experimental design, grant planning, or technology matching
- Fee-for-service options for organoid generation, phenotyping, and analysis
- Collaborative models for shared grant submissions and strategic partnerships
To get started, simply email us [ctindle@health.ucsd.edu] or visit our web portal for service request [link] to submit a project inquiry. A member of our team will follow up promptly to schedule a consultation and explore the best fit for your needs.
Contact Us
Courtney Tindle, M.S.
Humanoidcore@health.ucsd.edu