HUMANOID CoRE Mission
Reducing translational gap in drug discovery
Phase '0'
Clinical trials are failing patients. Drugs must go through a pipeline of triphasic testing before they can be approved by the US FDA: phase 1 to primarily assess safety; phase 2 to confirm safety and assess some efficacy; and phase 3, the pivotal efficacy trial. Most drugs fail somewhere along this pipeline, many in the last phase because of a primary lack of efficacy.
HUMANOID TM is pioneering a new approach to human clinical trials by introducing phase '0'. Before proceeding to patients in the clinic, phase 0 tests efficacy in multi-dimensional human organoid-based disease models-in-a-dish, where ineffective compounds can be rejected early in the process. Currently, most drugs are validated in two-dimensional (2D), cultured cells, which usually represent just one cell type. This simplistic model fails to recapitulate the multi-dimensional nature of human tissues where multiple cell types (epithelial, stromal, immune) interact in 3-dimensions within the extracellular matrix, and some tissues have additional dimensions such as coexisting microbes. Using patient-derived stem cells to grow organoids, HUMANOID TM seeks to change the game by reverse-engineering these complex multi-dimensional healthy and diseased human tissues, one cell at a time.
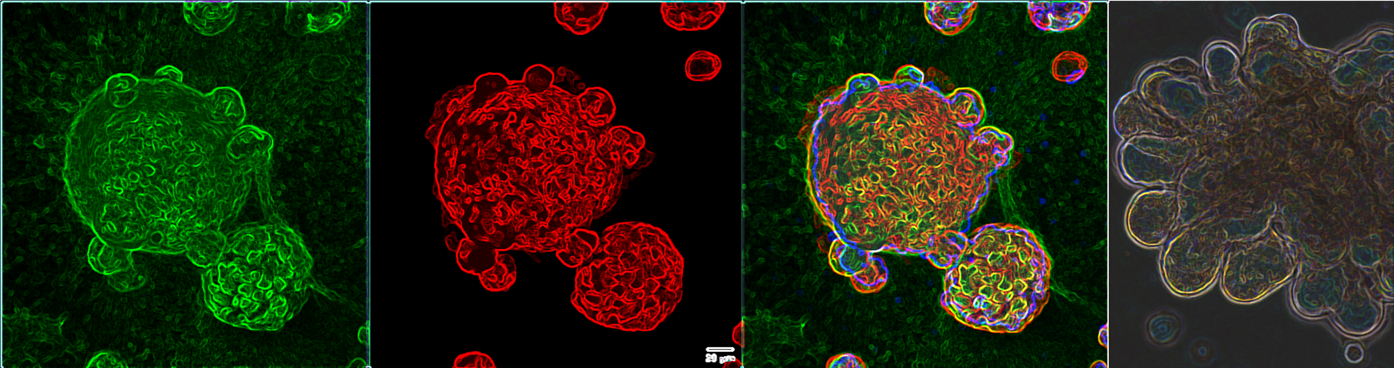
Organs-On-A-Dish
HUMANOID TM isolates, expand, and biobanks 3D organoids from healthy and diseased human tissues, isolates and biobanks primary cells and develops models to study organ physiology and human disease. Any tissue with stem cells can be grown as an organoid. Likewise, if a model requires microbes, immune cells, or non-immune support cells, HUMANOID TM researchers can incorporate them, constantly refining the model to achieve greater complexity, as needed, to emulate key aspects of the disease. These models are informed by human pathophysiology and validated by PreCSN's disease maps, translating engineered tissues/organoids into appropriate models to screen for drug efficacy and toxicity. Because organoids can be specifically derived from multiple patients in any disease subtype, these organoid-based models represent human organs-on-a-dish, enabling human phase 0 clinical trials.
How are HUMANOID Systems Created?
HUMANOID™ operates by a clearly defined ethos rooted in the principles of disruption through innovation, open science and collective progress. We don’t play by the rules; we make them.
Our signature program is BIODESIGN, embodies this spirit, modeling disease through four core principles:
🔹Embrace Interdisciplinary Integration: Our modeling philosophy is bidirectional: we simplify to understand, then reintroduce complexity to predict and test. Leveraging tools from engineering, computational science, and systems biology, we distill disease to its molecular essence—genes, proteins, metabolites—capturing the most outcome-deterministic features in streamlined 3D models. These models are then digitally reconstructed through in silico simulations to explore emergent behaviors and therapeutic interventions, anchored wherever possible to patient outcomes, imaging data, and epidemiologic trends.
🔹Model with Rigor, but Aim for Simplicity: Inspired by Picasso’s philosophy of abstraction—removing the superfluous to arrive at the essential—our BIODESIGN program applies computational abstraction and benchmarking to faithfully replicate core disease biology. By focusing on what truly matters, we build models that are both scientifically rigorous and clinically relevant.
🔹Anchor Models to Patient Outcomes: Many of our models are built from prospectively followed clinical samples, enabling direct correlation with patient trajectories. These models support "Phase 0" organoid trials that anticipate and inform later-phase clinical studies, accelerating translational impact.
🔹Reimagine Possibility: At HUMANOID™, we view uncertainty not as a barrier but as a catalyst for innovation. Grounded in clinical reality and fueled by imagination, we challenge assumptions and explore uncharted approaches—often uncovering answers others overlook.
Learn more about our BIODESIGN approach
Accelerate Drug Discovery
Early efforts have validated patient-derived organoids as effective platforms to screen for novel therapeutics in inflammatory bowel disease, microbe-driven polyposis in the colon, hereditary colorectal cancer syndromes, chemical-induced lung injury, and gastric cancer, as well as therapies that reprogram stem cells within solid tumors. Ongoing efforts are developing models for NASH/fatty liver and Alzheimer's disease. These models are rigorously vetted to validate their ability to replicate human disease. The next step will be multi-organ models to further capture human biology.
HUMANOID TM's organoids replicate critical aspects of human disease, offering more efficient ways to test potential drugs for toxicity and efficacy and giving researchers and clinicians new tools to conduct more efficient clinical studies. These organoids can also test existing drugs or multi-drug cocktails to help individual patients. Results take only days, giving physicians an incredible resource to prescribe personalized medicines for each patient's unique disease.
HUMANOID TM's organoid-based models provide a head start on efficacy, giving researchers an incredible tool to separate useful therapies from dead ends. This is how we accelerate the drug discovery process and bring new hope to patients.
Distinct Features of HUMANOID
Johns Hopkins began this movement in 1981, launching its Center for Alternatives to Animal Testing (CAAT), which formed a global alliance with CAAT-Europe. Its mission 3Rs: reduction, refinement, and replacement of animal research. HUB organoids were established in 2013 (HUB), which provided the technological leap, a foundation for creating living biobanks that are genetically stable, expandable, and cryopreservable. UC San Diego HUMANOID™ was established in 2019, with a singular goal, discipline end-to-end humanness of NAMs into simple and scalable models that maintain another set of 3Rs: reproducibility, relevance (e.g., to Phase 3 clinical end point) and regulatory-readiness (e.g., integrated into workflows for drug screens). At that time, human model research was considered hype. And yet, from choosing our tagline “Meet Us at Phase Zero” to trademarking HUMANOID™ systems, every step was intentional, because we saw organoids as foundational vessels for translation.